The oncology, hematology and acute/chronic disease development partner for biopharmaceutical sponsors
D2V Clinical is a full-service global CRO offering deep clinical development insight with world-class quality and investor value creation for biopharmaceutical sponsors.
Meet D2V Clinical
D2V Clinical was founded to advance novel therapies for unmet medical needs, using our experience, knowledge and passion for solving complex drug development problems. Our leaders are known for moving the industry forward in the complex areas of early-phase oncology, hematology and rare disease and bringing value to patients and stakeholders.
We connect your studies to the right sites and populations to conduct efficient and seamless studies across the globe.

- 20+ years in global drug development
- FDA and global biopharmaceutical development roles
- Studies across the US and China
- 80+ FDA PK/PD early-phase oncology reviews
- Designed 65+ early-phase oncology and hematology studies
- 100+ experiences including Ibrutinib, Brexpiprazole, and Durvalumab
- Access to
- 300 GCP sites/hospitals
- China hospital prevalence data for 400M patients and 1M+ PIs
Decades of experience paired with exceptional poise and oncology, hematology and rare disease knowledge
Expert team
World-class clinical development insight delivered with a hands-on approach
- 20 years of remarkable leadership in studies across the world including roles in pharma, biotech, academia and senior FDA review.
- Advise biopharmaceutical sponsors on the most direct routes to global approvals.
- Flexible, relationship-driven approach offering less bureaucracy, interaction with our senior team, interpersonal communication, and a passionate investment in our clients’ success.
End-to-end capabilities
From early strategy through regulatory submission
- Early planning to avoid issues and delays.
- Removing barriers across the development continuum.
- Strategic partnerships with leading medical centers and sites in the US and China.
- Streamlined digital innovation strategies including online patient reporting, remote monitoring and digital patient visits.
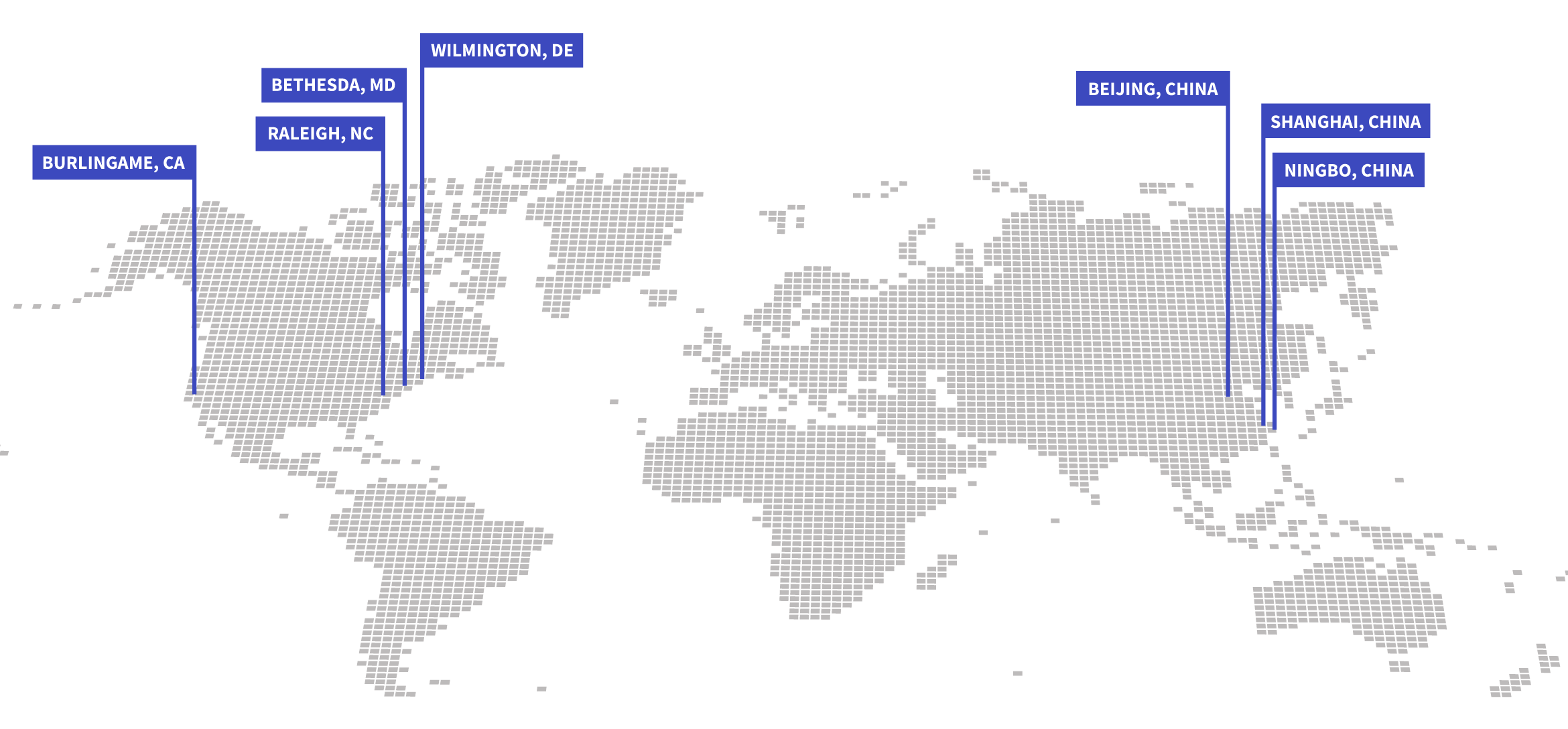
Bridge to/from US and China
Tap into a rich research landscape and vast patient population
- Connections that include KOL and site networks, top clinical experts, leading medical centers, PIs, regulatory authorities and patient networks.
- Dual track project management - sponsor local and enrollment region local to ensure seamless international communication.
Cancer and rare disease research across borders
We offer global capabilities and comprehensive solutions, resting on a foundation of clinical resources across the world. Our wide global reach allows biopharmaceutical sponsors to find the right sites and right patients, all with speed, efficiency and quality.

Our global footprint makes your research possible in the US and China