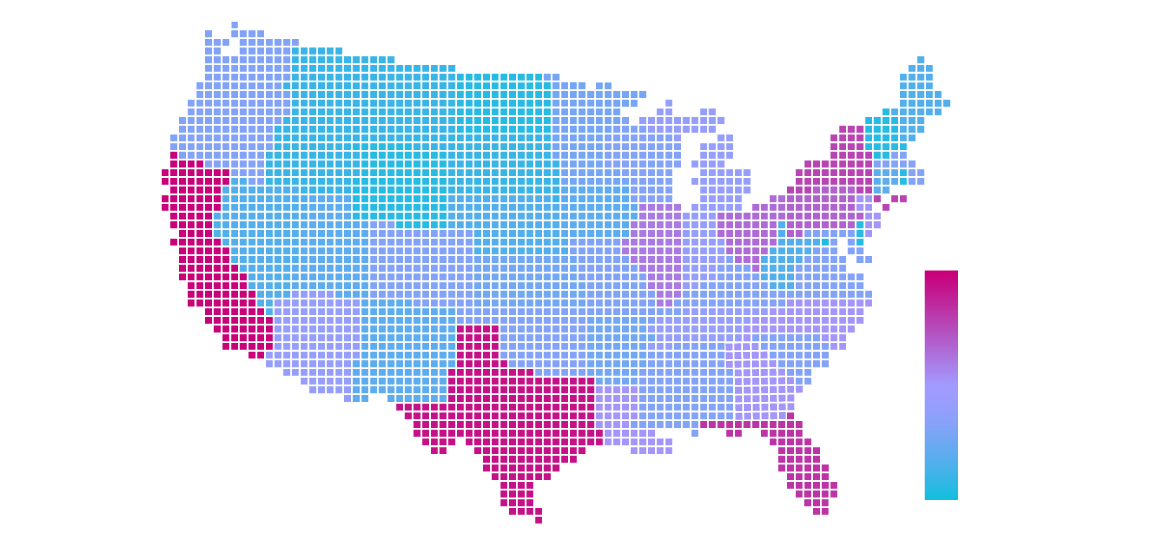
Our Centers and Sites
Advance your program in the US or China
Our exceptional team of experts has cultivated strong site relationships through 300+ clinical development programs and 20+ years of clinical development experience. With D2V Clinical, you can trust that your study will receive the prompt attention it deserves, setting the stage for a successful and timely clinical development journey.
We understand that study startup plays a pivotal role in meeting program timelines, so we approach it with unwavering urgency every single day, taking the following critical factors into account:

Site startup time

Site flexibility
D2V Clinical site support

Site performance
Our commitment to swift and efficient study startup processes allows us to minimize delays, optimize timelines, and ensure that our clients’ programs progress seamlessly.
Serving as your SMO in China
Site management – everything from study startup to patient screening, study coordination, monitoring, and QA – is essential to clinical trial success. However, in China, clinical trial sites and hospitals do not manage study delivery. To undertake clinical development in China, you must have a skilled site management organization (SMO) to serve as your local team.

D2V Clinical offers expert SMO services in China, maintaining quality, efficiency, safety, and regulatory compliance. Rely on our standard processes, best practices, and remarkable insight to help get your study to the finish line. Turn to us for study startup, site initiation, subject management, sample management, data management, file management, monitoring, quality assurance, communications, and close-out, and data archiving.